To understand the mechanism of electronic devices, it is imperative to understand its construction and material used in it. A Semiconductor is the core building block of electronic devices.
All the electronic devices and circuit in this world involves usage of a semiconductor.
To have a better understanding of electronic devices, it is imperative to understand semiconductor and to understand semiconductor we should be acquainted with materials.

You will get all the answers in this article. This primary vision behind this section is to make you familiar with the materials and its types.
Types of Materials
Materials are classified into three categories on the basis of electrical conductivity and that are Conductor, Insulator and Semiconductor. We will discuss all the three types of materials in detail. Besides, we will also discuss that on what factors their electric conductivity depends.
Conductor
Metals are Conductors; this is because of the electronic configuration of metals. The outermost electrons in the atoms of metal are loosely bound. At room temperature, these electrons can easily move in the space between the atoms.
Thus, the electrons in metals are called free electrons. This increases its conductivity as higher the relative mobility of electrons within a material; higher will be its electric conductivity. Thus, the movement of the electron is the fundamental term which decides its electrical conductivity.
Some examples of metals are- Silver, copper, gold, aluminium, iron, steel, etc.
Insulator
In Insulators, electrons in the outermost shell are tightly bound. At room temperature, the atom does not get sufficient heat energy to detach outermost electrons. Thus the electrons cannot move freely i.e. the electron mobility is almost zero. Therefore, its electric conductivity is also zero. Thus, the materials with extremely low electrical conductivity or no electrical conductivity are termed as insulators.
Some examples of Insulators are- Glass, rubber, plastic, paper, pure water, etc.
Semiconductor
Semiconductors are the materials with the electric conductivity less than conductors (metals) and more than insulators. In Semiconductor, the concentration level of free electrons lies between the density of electron in conductors and insulators.
Band Gap Theory
The Band Gap theory explains the mechanism of conductor, insulators and semiconductor regarding the movement of free electrons from valence band and the conduction band.
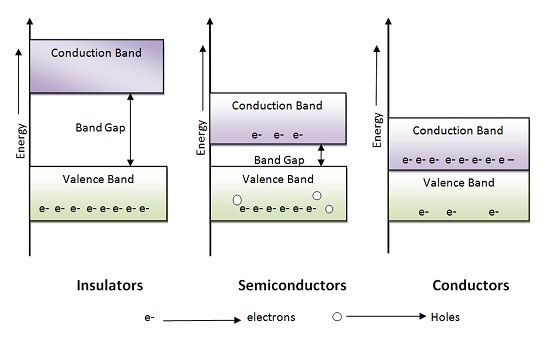
It is basically the energy band gap structure which clearly explains the conductivity of conductor, insulator and semiconductor. It comprises of two energy bands valence band, and the conduction band and the width between these two energy levels are termed as BAND GAP.
The electrons in the valence band are called valence electrons which are basically the charge carriers. If the band gap is more, the electrons in the valence band will require more energy to reach conduction band, in this way the conductivity of materials is manipulated.
Band Gap Theory Explanation for Insulator
In Insulators the difference between maximum energy level of the valence band and minimum energy level of the conduction band is very high. Thus, in the case of insulators, the width of the band gap is much is wider than that of conductor and semiconductor. Due to this electron in valence band require more energy to enter conduction band. On the other hand in the case of semiconductor and conductor, the magnitude of energy required is low.
Band Gap Theory Explanation for Conductor
In the case of conductors, the energy gap between valence band and conduction band is almost zero thus valence band, and conduction band overlaps. Due to overlapping electrons can easily flow from valence band to conduction band and contribute to electric conductivity.
Band Gap Theory Explanation for Semiconductor
In the case of a semiconductor, the energy level difference between the valence band and conduction band is comparative that insulators, but even at room temperature electrons get sufficient energy to enter conduction band.
The Energy Band Gap in the case of Germanium is 0.67eV at 302K and band gap in case of Silicon is 1.11 eV at 302K.
The electrons can move freely in semiconductor if they are provided with a small amount of energy thus even at room temperature semiconductor acts as a conductor and at temperatures lower than room temperature it acts as an insulator.
Electron-Hole Pair
When electrons move from valence band to conduction band, it leaves a hole behind it in valence band which can be occupied by other electrons in the valence band. Holes are positive charge carriers. In a semiconductor, the number of holes is same as that of electrons. Thus, for every electron moving in conduction band there will be a hole in the valence band.
Thus, the semiconductor as a whole is neutral. At room temperature, there is about 1010 free electrons in the conduction band and holes in the valence band. But the electrons always occupy lowest energy state thus if there is no energy supply they fall back into the valence band.
At a particular temperature, equilibrium is established between the electrons entering conduction band and the electrons which are falling to the valence band. With the increase in temperature more electrons transit from the valence band to conduction band and thus the conductivity of semiconductors increases.
Leave a Reply