Semiconductor shows this behaviour due to their electronic Configuration. In addition to this, they exhibit specific properties according to their unique position in the periodic table. Group – IV elements in the periodic table are used as Semiconductor. Germanium and Silicon are categorised in Group – IV of the periodic table.
The common property of all these elements is that they have 4 electrons in the outermost shell. The atoms of these materials have a particular structure, and this structure is periodic in nature. Thus, it repeats itself continuously. One complete pattern is called crystal, and the periodic arrangement of atoms is called lattice.
According to the atomic configuration of Germanium and Silicon both have 4 electrons in their outermost shell. These electrons are valence electrons. But an atom requires 8 electrons in its outermost shell to attain stability. Atoms of Inert gases are stable like Argon, Neon, Xenon, Krypton because they have 8 electrons in their outermost shell. Likewise, Germanium and Silicons tends to acquire 8 electrons to achieve stability
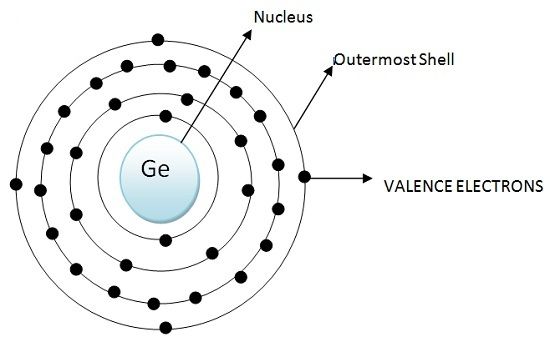
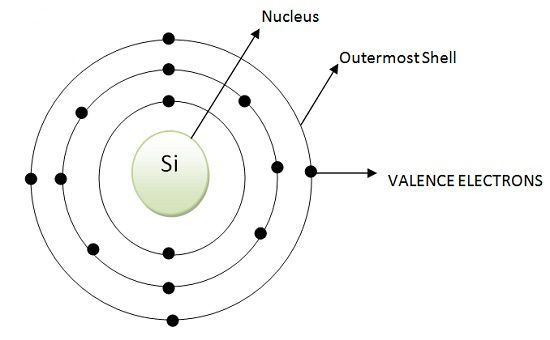
The atomic configuration of silicon is shown below:
To gain four more electrons, they form covalent bonds with neighbouring atoms. But the strength of the covalent bond is much lower than the ionic bond. Thus, covalent bonds are weak. Therefore, even small amount of kinetic energy can break these bonds.
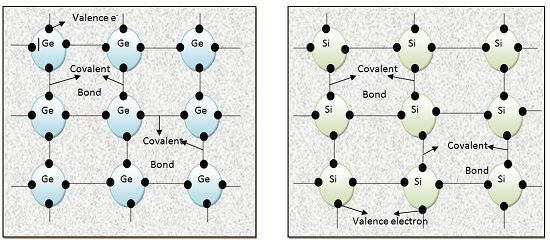
That’s why forbidden energy gap in case of Semiconductor is small. If a small amount of heat energy or light energy is provided to semiconductor crystal, it starts conducting. This is because kinetic energy imparted to it through heat or light is sufficient to break covalent bonds. When these bonds break electrons become mobile charge carriers.
Intrinsic Semiconductor
More the free electrons more will be the mobility and higher will be the electric conductivity.These are the pure semiconductors, and it is also called Intrinsic Semiconductor. It possesses negative temperature coefficient. Thus, it means its resistance will decrease with increase in temperature, and hence electric conductivity will increase. Thus, intrinsic semiconductors will conduct only when heat or light energy is imparted to them.
Extrinsic Semiconductor
When impurity atoms are added to an intrinsic or pure semiconductor, it becomes Extrinsic Semiconductor. The properties of Semiconductor is varied by adding impurity atom. This is called doping, i.e., adding impurity deliberately to improve conductivity. One impurity atom is added to 10 million atoms of an intrinsic semiconductor.
Extrinsic Semiconductors can be further classified into two types i.e. N-type semiconductor or P-type Semiconductor. It depends upon the nature of impurity atom added.
1. N- type Semiconductor: It is formed when the pentavalent impurity is added to an intrinsic semiconductor. The pentavalent material is categorised in Group – V of the periodic table. These materials have 5 electrons in their outermost shell. Therefore, they are called pentavalent material. Pentavalent impurities are Phosphorus, Antimony, Arsenic, etc.
2. P-type Semiconductor: It is formed when the trivalent impurity is added to an intrinsic semiconductor. Trivalent materials are categorised in Group – III of the periodic table. These materials have 3 electrons in their outermost shell. Therefore, they are called trivalent material. Trivalent impurities are Boron, Gallium, Indium, etc.
![]()
You can understand this with the help of construction of N-type and P-type semiconductor.
Construction of N-type Semiconductors
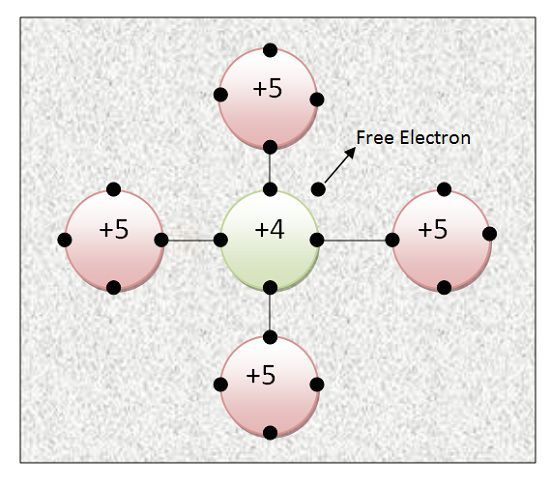
When Pentavalent impurity atom is added to Pure semiconductor its conductivity increases. Let us consider Arsenic is added to Germanium. The five outermost electrons of Arsenic will try to acquire stable state. For acquiring a stable state, they need 8 electrons in the outermost shell.
Thus, out of 5 electrons of outermost shell of Arsenic, 4 electrons will form the covalent bond with outermost 4 electrons of Germanium and acquire stability. The remaining one electron is the free electron. Arsenic atom will donate this single electron.
Thus, Pentavalent impurities are called donor impurities. This free electron will take part in conduction and increase conductivity. In this way for every covalent bond, there will be one electron in excess. Thus, it has electron as majority charge carriers. Electrons are negatively charged particles. Thus, it is called N-type i.e. Negative type Semiconductors.
It also has positive charge ions. When donor impurities donate one electron to the crystal, it becomes a positive ion with one +ve charge because it has donated one electron. Thus, N – type semiconductor has electrons as majority charge carriers and ions (positively charged) as minority charge carriers.
Construction of P – type Semiconductors
When trivalent impurity atoms are added to Pure Semiconductor, its conductivity shows a significant increase. In this case, electrons are not majority charge carriers here. Let us consider one atom of Gallium is added to 10 million parts of Silicon. The three outermost electrons of Gallium will try to acquire stability. For acquiring stability, they need 8 electrons in the outermost shell. But Silicon has only 4 electrons in its outermost shell.
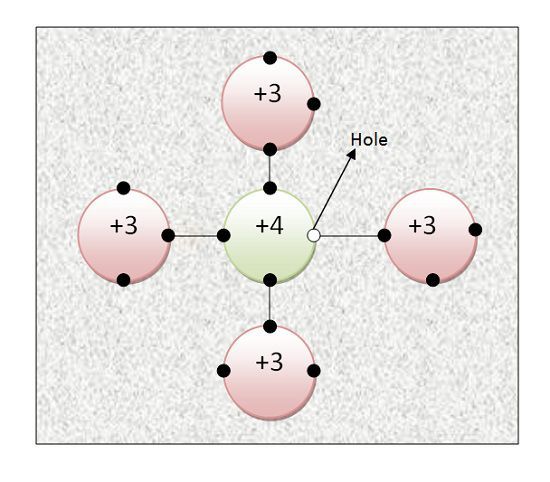
So, 3 electrons of Gallium atom will form covalent bonds with 3 electrons of Silicon atom. One electron of Silicon atom is left. It cannot become free electron because neither Silicon atom has attained stability nor Gallium.
The trivalent impurity is known as acceptor impurities. So, this electron will try to form the covalent bond with Gallium. Since Gallium does not have an extra electron so the covalent bond is devoid of an electron.This devoid of an electron is considered as holes. Holes are positive charge carrier.
Thus, P-type Semiconductors has majority charge carriers as holes and electrons as minority charge carriers. This is the reason it is called P-type semiconductors or Positive type Semiconductors. Holes are responsible for conduction in the P-type semiconductor.
Akbar says
Is there any further classification of intrinsic semiconductors?
Roshni Y says
No, intrinsic or un-doped semiconductors are not classified further.