Definition: Thermionic effect or Thermionic Emission can be defined as the phenomenon in which electrons are emitted from the surface of the metal when heat energy is applied to the metal. The word Thermionic is formed from the words Thermal and ions. Thermal means heat and ions are charged particles.
Thermionic emission depends on three factors, temperature of the metal surface, area of the metal surface and last but not the least the work function of the metal.
Significance of Thermionic effect in Electronics
The thermionic effect holds a vital position in operation of some crucial electronic devices. For example, let’s consider a cathode ray tube. The principle of working of cathode ray tube revolves around the hot cathode which is nothing other than the by-product of thermionic effect.
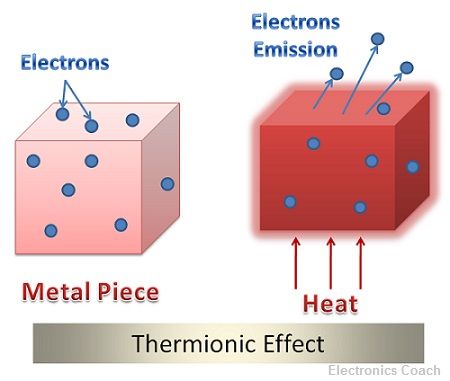
The emitter or the hot metal surface which emits electrons after supplying heat energy is termed as hot cathode ray tube. Thus, thermionic effect forms the basis of various electronic devices.
The entire arrangement of emitter metal is placed inside the evacuated tube or tube filled with inert gases. If it is placed in the open atmosphere, the metal which is heated to high temperature will react with the oxygen present in the atmosphere. This leads to deterioration of the metal.
Thus, if it is placed in evacuated space or space filled with inert gases, the metal will be preserved because inert gases do not react with any metal element due to their complete atomic configuration.
Mathematical Explanation of Thermionic Effect
The electrons emitted during the process of thermionic emission depends upon the surface area of the metal surface and the temperature of the surface. It can be expressed mathematically with the help of equation given by O. W. Richardson Dushman.
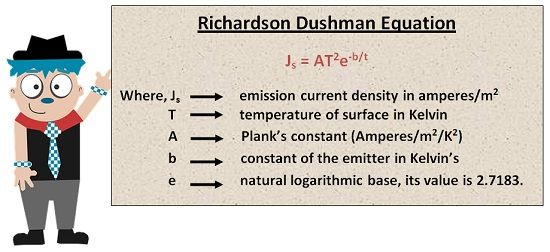
The value of proportionality constant which is also termed as Plank’s Constant is 600,000 for most of the metals, but it can vary with the varying characteristics of the metal. The below equation can determine the value of b. It is dependant on the particular features of the metal. Thus, it is constant for a particular metal, but it shows variation with varying temperature.

Thus from the above equation, it is clear that to achieve maximum emission, we need to have two things. Either works function of the metal should be low, or the temperature of the metal surface should be high. If we can achieve the conditions mentioned above, a significant number of electrons will leave valence band and enter into the vacuum.
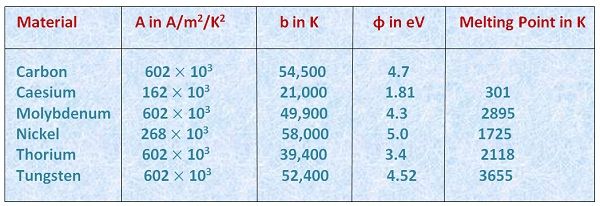
The values of these constant for some metals are given in the table below. Besides, the maximum current which flows at a particular temperature is called the saturation value of the temperature.
Understanding Thermionic Effect with the help of Analogy
Consider an example of boiling water; the water will start boiling, only when the heat energy applied to it will be sufficiently high. If the applied heat energy is not high enough to attain its boiling point, the water will not boil.
Similar to the boiling point of water, metals too, have threshold temperature below this temperature none of the electrons will emit. On the contrary, when the temperature becomes equivalent to or greater than the threshold temperature, the metal will emit an electron.
The boiling point is nothing but the threshold temperature above which the water starts boiling. Similarly, threshold temperature for thermionic emission is the temperature above which thermionic effect can be observed.
The electrons present in the valence shell are bonded with the atom. This is due to the force of attraction exerted on them by the nucleus of an atom. To release the electrons from the valence shell, the energy applied from the external source should be sufficiently high, to overcome the force exerted by nuclei of an atom.
The emission particles are known as thermions. The emission of these thermions is dependent on certain factors. Let’s put light on the factors controlling thermionic effect.
Factors Influencing Thermionic Effect
- Work Function of the Metal: The work function of the metal is the minimum energy required by the atom of the metal to emit an electron. The lower value of work function means it is easy to provide emission of an electron from the atom of that metal. Higher work function means the metal requires high energy to provide emission.
Thus, the rate of emission of thermions can be increased, if the metal with low work function is utilised for this purpose.
2. The temperature of the Surface: The temperature of the surface plays a vital role for thermionic emission. If the temperature of the emitter metal is high, more electrons will be released. As high temperature provides thermal energy to the electron which leads to the increment in their kinetic energy.
3. Surface Area of the Metal Surface: Higher the surface area of the metal, higher will be the thermionic emission. This is because more the surface area more will be the rate of emission. The large surface area provides more space for electrons to emit. Thus, if large surface area metals are used the rate of thermionic emission will increase proportionally.
Variation of thermionic Current Density with Temperature
The diagram below illustrates the variation of thermionic current density with the temperature of the metal emitter.
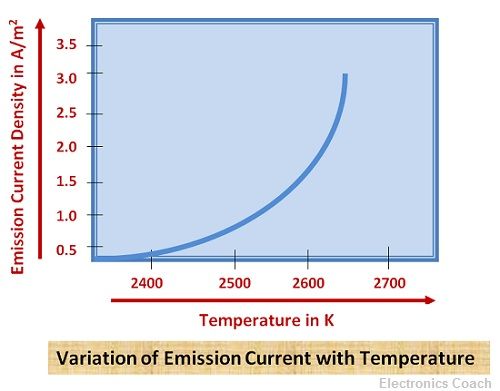
It is clear from the above graph that the emission current density varies linearly with the increase in temperature.
rinku says
Awesome
Felix says
The site is awesome.